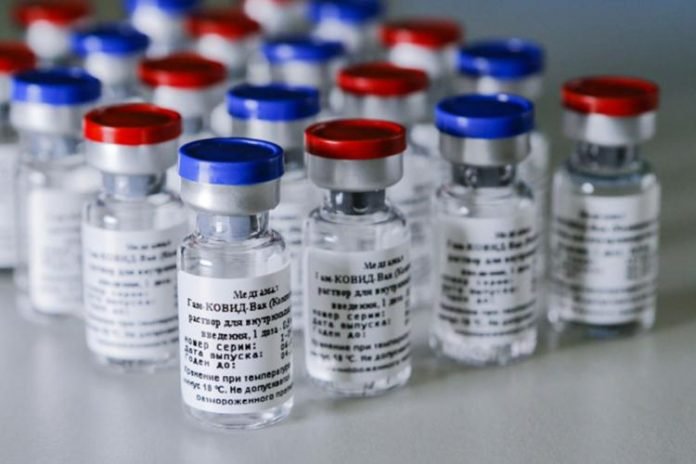
India’s Drug Regulator-Drug Controller General of India (DCGI) has accepted the recommendations of the Expert Committee on Vaccine. That is, now the vaccines of Moderna, Pfizer and Johnson & Johnson (J&J) will be approved soon.
The National Expert Group on Vaccine Administration for COVID-19 (NEGVAC) suggested importing the approved vaccine from the US, Europe, UK, Japan, and the World Health Organization (WHO). The Drug Controller General of India VG Somani has issued the terms and procedure to approve these vaccines for emergency use. Vaccination started on 16 January in India. Then Kovaxin of Bharat Biotech and KoviShield of Serum Institute of India began to be used. Last week, India’s drug regulator has also approved the Russian vaccine Sputnik V. As soon as the approval for foreign vaccine is opened, the lack of vaccine dose in the country will help to overcome the bottlenecks in the path of vaccine for everyone.
How will foreign vaccine be allowed?
The foreign vaccine will be first introduced to 100 people. Then an attempt will be made to know whether the vaccine is safe or not. These 100 people will be monitored for seven days. It will then be decided whether to approve the emergency for the vaccine or not.
If the vaccine proves to be safe, it will receive emergency approval, but with one condition. Vaccines that have been trialed abroad have to undergo a bridge trial in India. In this, like phase-3 clinical trials, thousands of volunteers are not given doses, but a few hundred people have to prove the results abroad through trials. The trials of Kovyshield and Sputnik V have happened in the same way.
Under the new rule, if the foreign vaccine is found safe in seven, it will be approved on trials mode. That is, the person who will be given the dose of the vaccine will get the consent form filled. As it initially happened to those applying covaxin. When the results were out, this cleanup trial mode was removed. Similarly, when the results of the bridge trials come, then the bridge trials mode will be removed from the foreign vaccine.
Which vaccine can be approved?
According to the new decision, only the US use of US regulator USFDA, EU regulator EMA, UK regulator UK MHRA, Japan’s regulator PMDA and WHO in the list of emergency use listings will be given emergency use approval in India.
At present, only Johnson & Johnson’s vaccine approval has been approved with Moderna, Pfizer in the US. Similarly, in addition to these three in the European Union, AstraZeneca’s vaccine has been approved. Pfizer, Moderna and AstraZeneca vaccines are being installed in the UK. Only Pfizer vaccine in Japan. The WHO has so far approved only two vaccines – Pfizer and AstraZeneca.
In such a situation, the vaccines of Pfizer, Moderna and Johnson & Johnson are such, which are being used in these countries and not with us. These vaccines can be considered to be an emergency approval in India.